A Weizmann Institute of Science study shows how a deadly virus gets into cells
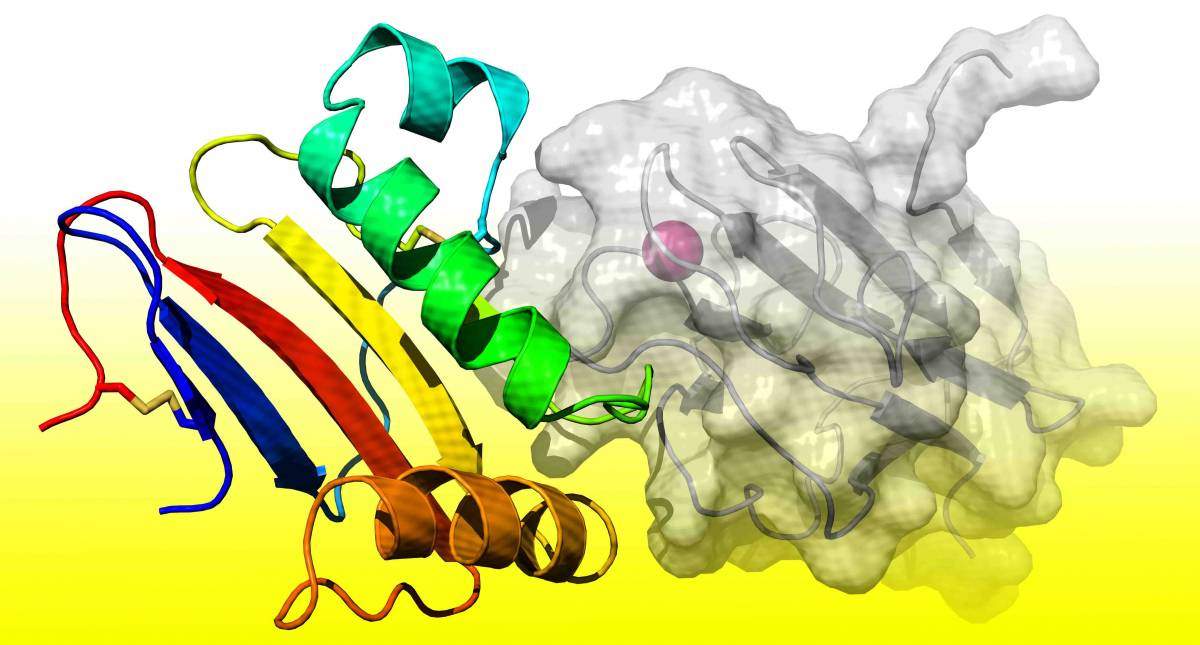
The GP1 receptor binding domain of Lujo virus (rainbow-colored ribbon) bound to its NRP2 receptor (grey ribbon and surface representations). A Ca2+ ion needed for the formation of this comple
When a virus infects a new host, it must find the proper host cells, attaching to and then breaching their membrane walls to get its viral genetic material inside for replication. Dr. Ron Diskin and his group in the Weizmann Institute of Science recently revealed the unique mechanism that one particular virus uses to locate and bind to its target cell. Their findings were reported in Nature Microbiology.
The virus, Lujo virus, was first identified in 2008 in southern Africa, when four of the five people known to be infected died of the disease. Diskin, of the Institute’s Structural Biology Department, researches viral pathogens like this that mostly live in animal hosts, occasionally crossing over to humans. In the case of Lujo, which is a small RNA virus like influenza or Ebola, the animal in question is not known, but the disease can be transmitted between humans, it is highly deadly and there is no vaccine or cure. “Almost no one has heard of this virus, but for all we know, it could be at the center of the next epidemic,” he says. “People move from place to place rapidly these days, helping diseases spread. And some think that in the future, if animals relocate due to climate change, more humans could be affected by such zoonotic diseases.” Lujo virus has since been identified as a member of a family known as arenaviruses, a group that includes the better known Lassa virus, which causes a deadly hemorrhagic fever.
Around a year ago, a group of researchers identified the Lujo virus’s port of entry into the human cell. Like nearly all viruses, Lujo attaches itself to a protein complex called a receptor on the cell’s outer membrane – like a cleverly-faked chemical ID card – initiating a process that allows it to fuse its viral membrane with the cell membrane. But the particular receptor the researchers had identified was new to them – it was not used by other known arenaviruses.
Combining strands into crystals

Dr. Ron Diskin and Dr. Hadas Cohen-Dvashi showed how one virus obtains the recognition it seeks
Diskin, Dr. Hadas Cohen-Dvashi from his group and former student Itay Kilimnik revealed the exact details of the process by which the cell receptor and Lujo virus recognize one another and bind. They first obtained a part of the virus – a subdomain of the trimeric (triple-strand) complex known as GP1, which is known to be involved in recognizing the receptor. The researchers then combined these bits of the virus with the parts of the receptor involved in the recognition and binding process. Finally, they turned whole complexes – viral proteins together with receptors – into crystals that could be examined by X-ray crystallography.
Not only was the receptor new, its binding method was also different from the others. Most surprising to the researchers was the fact that the binding site on the receptor had a calcium ion facilitating the process; when the researchers removed the ion, binding did not occur. Although this sort of binding occurs between proteins in the body, it is rare in virus-receptor interactions. The researchers were also surprised to note that the GP1 receptor-binding module did not change its configuration. Other arenavirus GP1 subdomains like those in Lassa do change configuration when produced apart from their entire trimeric complexes. This observation hints at the possibility of utilizing GP1 domains to produce anti-Lujo immunotherapy.
A complex that stays in shape
The researchers conducted further investigations with “pseudo-viruses” – artificial partial viruses they created in the lab by inserting the complete spike complex of Lujo virus onto a viral envelope containing genes that fluoresce in different colors under a microscope, but minus the rest of the infectious genome. With these, they were able to confirm the roles that the various parts of the viral spike and receptor play in recognition and binding.
Among other things, the study showed that the surface of the cellular receptor the Lujo virus uses as an entryway is completely conserved in nearly all mammals, explaining the ability of the virus to efficiently transmit to humans. This also means the animal host may remain a mystery for the present. The good news is that the discovery of the unique features may help point to the development of a treatment, and since the GP1 complex keeps its shape, neutralizing antibodies to suppress Lujo might do their job more easily.
The cellular receptor the Lujo virus uses is conserved in nearly all mammals, explaining the ability of the virus to efficiently transmit to humans
The study showed that Lujo is closer on the evolutionary tree to the Old World group of arenaviruses; this group has been found, for reasons that are still not completely clear, to be particularly resistant to the body’s antibody-based immune response. “That doesn’t mean a vaccine cannot be developed, but we may have to look beyond the simple antibody model in order to create one,” says Diskin. Although his lab does not have the biological safeguards needed to study the live infection process, he and his group have ongoing collaborations with several labs in the US that do. “Beyond elucidating the basic molecular mechanism that the Lujo virus uses to locate its cellular target, the findings of our study will hopefully assist in the development of treatments or a vaccine that would make us better prepared for a potential future outbreak of this deadly pathogen,” he says.
Dr. Ron Diskin’s research is supported by the Moross Integrated Cancer Center; the Lester Crown Brain Research Fund; and the estate of Emile Mimran.

Recent Comments