The new method may enable quality control without costly purification procedures
Turning weakness into strength is a strategy for success championed by personal growth coaches and business consultants. The group of Prof. Michal Sharon in the Biomolecular Sciences Department of the Weizmann Institute of Science has applied this stratagem to the analysis of proteins. As reported in Communications Biology, the result is an approach that immensely speeds up quality control in the production of proteins manufactured for use as drugs and for a wide variety of other applications.
In Sharon’s lab, proteins are analyzed by highly advanced versions of mass spectrometry, making it possible to determine a protein’s makeup down to the atom. But the technique is thought to have a major limitation: When the contents of entire cells are analyzed, proteins produced in larger than usual amounts in these cells overshadow all the others, causing the other proteins to remain practically invisible.
This surely is a drawback if one wants to obtain information about each and every protein in the cell. But scientists in Sharon’s group thought about the situation in which a protein is deliberately overproduced and is the only one of interest. That’s the case for recombinant proteins, those produced by DNA manipulation technologies: The gene for a protein is inserted into a small DNA molecule inside a bacterium, yeast or an animal or human cell, which then starts making this protein in large amounts. To make sure a recombinant protein meets the required standards and to optimize its production, its quality needs to be assessed at various stages of the production process. To this end, the host microorganism or cell is destroyed, and the protein is purified before being subjected to analysis.

(l-r) Prof. Michal Sharon, Dr. Gili Ben-Nissan and Shay Vimer
This is precisely the step for which Sharon’s group had the idea to turn a weakness into a strength. If the overproduced protein is so dominant, perhaps the others will be, for all intents and purposes, invisible, and all the proteins from the host microorganism or cell can be directly subjected to analysis by mass spectrometry, skipping the costly and labor-intensive purification process?
Dr. Gili Ben-Nissan and Shay Vimer, together with other members of Sharon’s team, conducted a series of experiments in which they genetically manipulated yeast, as well as insect and human cells to each overproduce one recombinant protein. They then applied mass spectrometry to these proteins, in purified and non-purified form, and compared the results.
The analysis worked equally well in both scenarios – that is, when the protein had been purified prior to analysis and when it hadn’t. In both cases, the scientists were able to assess plenty of the protein’s characteristics, including its molecular weight, folding, solubility, assembly state, overall structure and even its ability to bind to other relevant biological molecules. It took them as little as 30 minutes to obtain this information without prior purification, whereas obtaining the same data following purification can take up to two or three days.
These results showed that characterizing recombinant proteins by mass spectrometry without prior purification can enormously simplify and speed up their quality assessment. This finding is particularly valuable in view of the recent dramatic increase in the use of recombinant proteins. The first recombinant protein drug was insulin, produced back in 1982; since then, dozens of others have been generated. Since 2011, for example, more than 60 recombinant protein drugs have been approved by the Food and Drug Administration in the United States, including those intended to diagnose or treat cancer, autoimmune and genetic disorders, and infectious diseases. Examples include belimubab, an antibody approved for the treatment of lupus, glucarpidase, an enzyme given to cancer patients with impaired kidney function, and tbo-filgrastim, a growth factor that stimulates the production of white blood cells. In other areas, recombinant proteins include the vast majority of industrial enzymes used in the production of paper, leather, detergents, textiles, biofuels and food. In the food industry, at least one recombinant protein serves as an artificial sweetener; others are used to facilitate coffee processing and the manufacture of fruit and vegetable juices, among numerous additional uses.
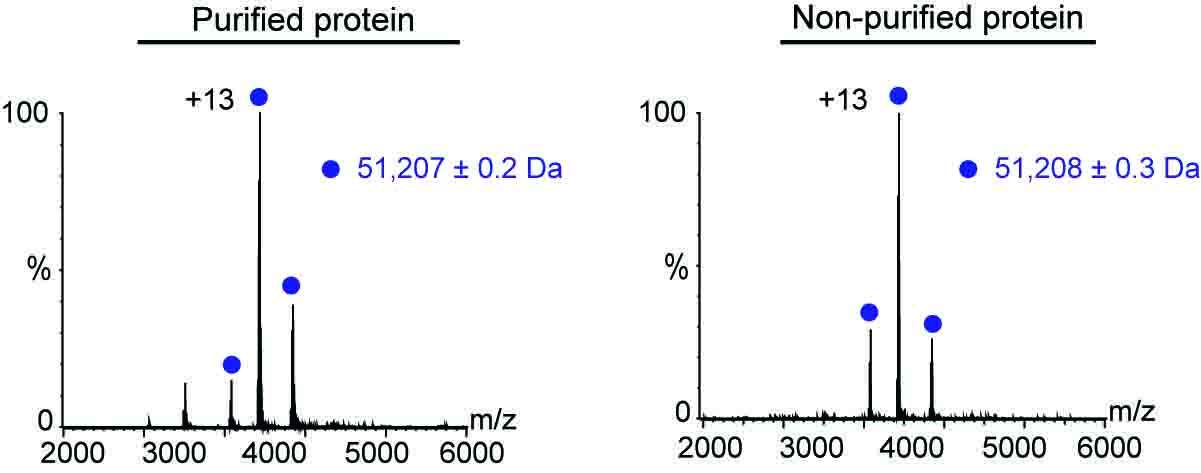
Mass measurements for the purified and non-purified protein were almost identical
Study participants included Dr. Shira Warszawski and Dr. Sarel J. Fleishman of the Biomolecular Sciences Department; Aliza Katz, Dr. Hadas Cohen-Dvashi and Dr. Ron Diskin of the Structural Biology Department; Meital Yona, Dr. Tamar Unger and Dr. Yoav Peleg of the Life Sciences Core Facilities Department; and Dr. David Morgenstern of the Nancy and Stephen Grand Israel National Center for Personalized Medicine.
Prof. Michal Sharon’s research is supported by the Benoziyo Fund for the Advancement of Science; Karen Siem; and the Estate of Zvia Zeroni. Prof. Sharon is the incumbent of the Aharon and Ephraim Katzir Memorial Professorial Chair in Translational Research.

Recent Comments