Zombie-like cells play a role in repair – if they are properly regulate
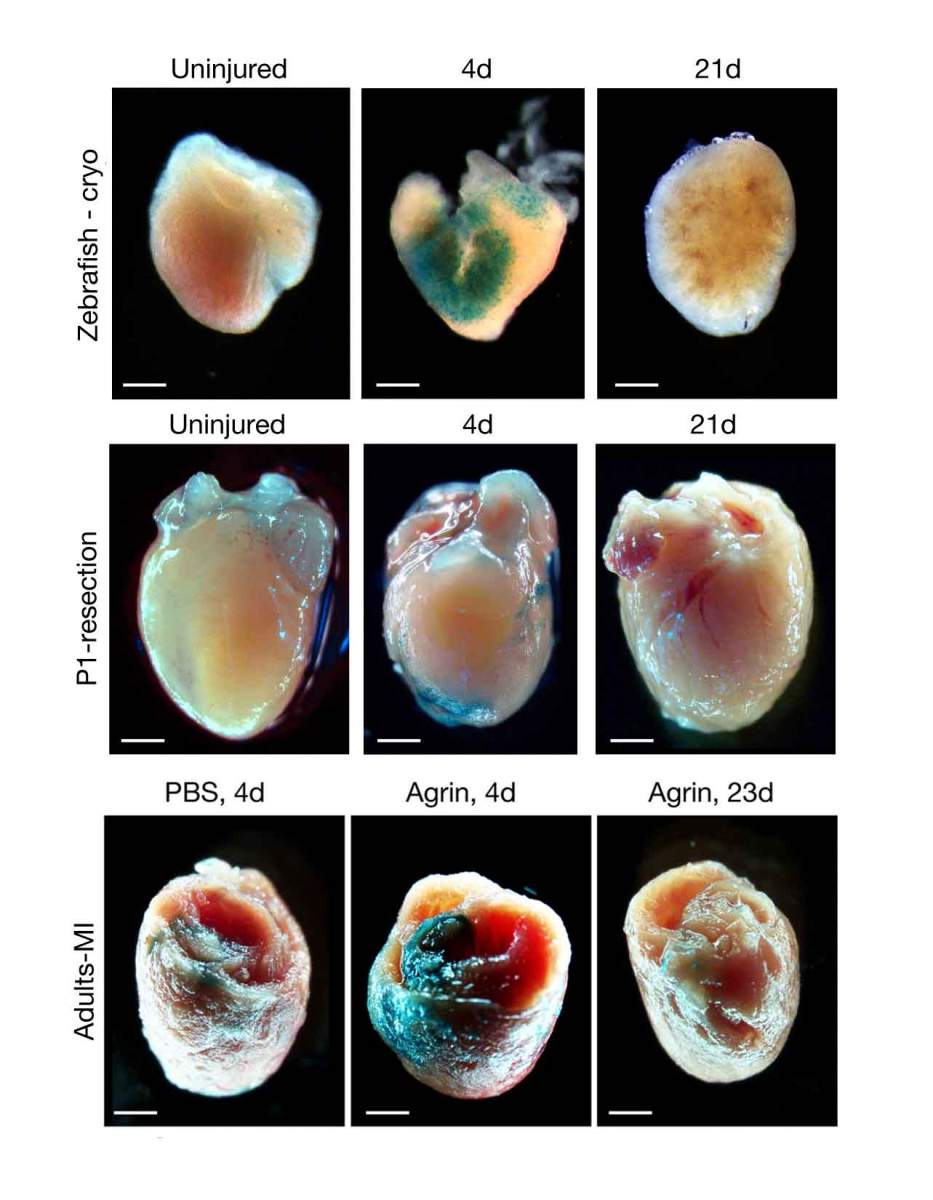
Transient senescence: The hearts of zebrafish (top row), newborn mouse (middle row) and adult mouse (bottom row) contain hardly any senescent fibroblasts (cyan) prior to injury (left column). In the wake of an injury (followed by Agrin injections in the case of the adult mouse heart), numerous fibroblasts enter a senescent state (middle column), but are cleared from the heart within about three weeks (right column)
Since cardiac muscle cells are the main performers in keeping the heart pumping, it is no wonder that research efforts aimed at repairing this organ have focused on heart muscle cells. For example, major efforts are aimed at trying to create new such cells or to coax the existing ones into regeneration. But a new Weizmann Institute of Science study published recently in Circulation puts the spotlight on a less glamorous group of cells in the heart – the fibroblasts, which used to be known mainly for their supporting role in providing the underlying structure for the muscle but are now emerging as potential major players in heart repair.
Staff scientist Dr. Rachel Sarig, working in the laboratory of Prof. Eldad Tzahor of the Molecular Cell Biology Department, investigated situations in which the heart muscle is expected to regenerate – for example, in newborn mice. She discovered that in such situations the fibroblasts, which make up about half of all heart muscle tissue, temporarily enter a zombie-like state known as senescence, in which they neither die nor divide. With the help of MSc student Racheli Rimmer and other team members, Sarig found evidence suggesting that this transient state, which the scientists called “regenerative senescence,” may be facilitating repair.

(l-r) Dr. Rachel Sarig, Prof. Eldad Tzahor and Racheli Rimmer
Essential to renewal
Senescence is commonly associated with aging and disease but in its transient form, it is known to be essential for proper fetal development, as well as for the regeneration of organs in such creatures as salamanders. The Weizmann study is the first to reveal a transient senescence phase that occurs in the heart. The researchers observed it in three situations characterized by heart muscle regeneration after injury: in the hearts of adult zebrafish, in the hearts of newborn mice, and after they’d injected into injured hearts of adult mice a protein called Agrin, which had been shown to trigger heart muscle regeneration in earlier research in Tzahor’s lab. In all these cases, the signs of senescence disappeared after three weeks. “Senescent fibroblasts can promote repair by releasing growth factors and other molecules that stimulate healing, and by recruiting immune cells that may also facilitate the repair,” Sarig says. “But if senescence persists for too long, it can cause chronic inflammation and other unwanted effects, so it needs to be tightly regulated – a proper balance is crucial.”
The timing of the senescence – the fact that it occurred in each case simultaneously with heart regeneration – suggests that this transient state is just as essential to heart muscle renewal as it is in other instances of tissue regrowth. The importance of this regenerative senescence is further reinforced by the fact that it has been conserved in the course of evolution, from zebrafish to mammals.
Genome guardian regulates transient senescence
The scientists found that transient senescence in the heart muscle appears to be regulated by the p53 gene, the tumor suppressor that is known as “the guardian of the genome” but which also plays other roles in the cell. Activity levels of a particular version of the p53 gene increased sharply precisely at the time that transient senescence occurred.
If senescence persists for too long, it can cause chronic inflammation and other unwanted effects
“Our results suggest that p53 activity may serve as a marker of transient senescence in the heart muscle,” Tzahor says. “When we learn more about the mechanisms of this kind of senescence, it may become possible to aim for controlling this process in order to promote heart muscle repair.”
Although further research is needed, the scientists are hopeful that understanding the mechanisms of regenerative senescence may lead to new approaches to repair the heart muscle.
Also involved in this study was Prof. Karina Yaniv of the Biological Regulation Department.
Prof. Eldad Tzahor’s research is supported by the Yad Abraham Research Center for Cancer Diagnostics and Therapy; the Zuckerman STEM Leadership Program; the Dvora and Haim Teitelbaum Endowment Fund; the Daniel S. Shapiro Cardiovascular Research Fund; the European Research Council; and Pearl C. Vapnek.
Dr. Rachel Sarig is the incumbent of the Gabriella Schmidt Research Fellow Chair.

Recent Comments